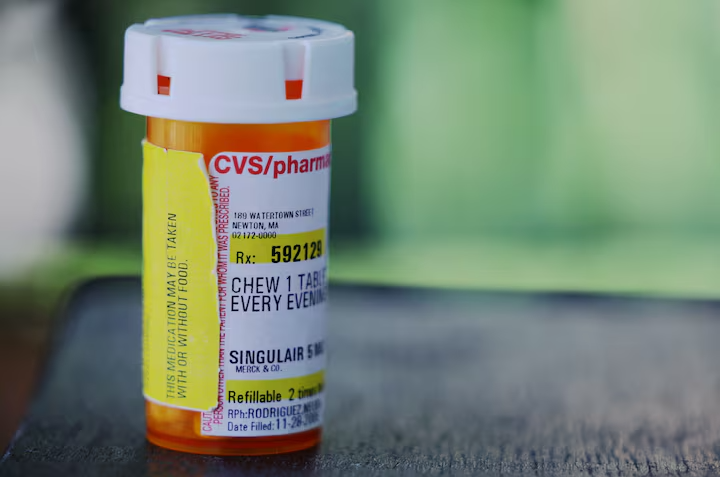
New research by U.S. government scientists has uncovered evidence suggesting that montelukast, a widely used asthma and allergy drug sold under the brand name Singulair, may bind to brain receptors critical for psychiatric functioning. Laboratory tests indicate that the drug, which has been prescribed to millions globally, accumulates in brain regions linked to mood, cognition, and sleep regulation. Although the findings are preliminary, they were presented at a toxicology conference, raising concerns about the drug's safety profile.
Originally launched by Merck in 1998 as a breakthrough oral alternative to inhalers, Singulair was marketed as having minimal side effects. However, by 2019, thousands of reports connected the drug to neuropsychiatric episodes, including depression, suicidal thoughts, and behaviors. The U.S. Food and Drug Administration (FDA) responded by adding a "black box" warning to the drug label in 2020, flagging potential serious mental health risks. Despite mounting evidence, the FDA has no plans to update the label further based on the latest research.
Jessica Oliphant, an FDA official, highlighted that montelukast showed significant binding to receptors in the brain during lab tests, similar to other drugs known for psychiatric side effects. This aligns with prior studies demonstrating the drug's penetration into the brains of test animals. Austrian researchers Julia Marschallinger and Ludwig Aigner further confirmed the drug’s presence in brain regions regulating impulse control and mood, although they acknowledged the need for more research to identify which patients may be at greatest risk.
Reports from families of patients underscore the potential dangers. For instance, Robert England described how his 22-year-old son Nick tragically took his own life just days after beginning montelukast treatment. England said his son had no prior mental health issues, and his sudden behavioral changes were alarming. Since 1998, the FDA has recorded at least 82 suicides linked to montelukast, including 31 cases involving individuals aged 19 or younger.
Organon, a Merck spinoff that currently markets Singulair, maintains confidence in the drug’s safety and insists its label adequately warns of risks. However, ongoing lawsuits allege that Merck downplayed the psychiatric side effects in its early communications with regulators. As scientific investigations continue, questions remain about the drug’s risks, particularly for vulnerable populations.